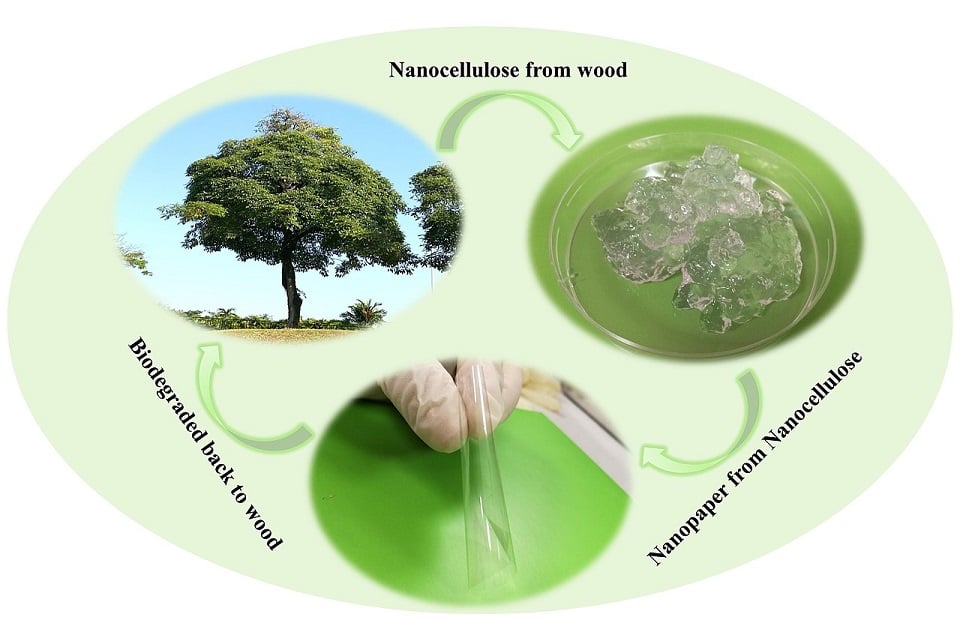
Nanocellulose is a term referring to nano-structured cellulose. This may be either cellulose nanocrystal (CNC or NCC), cellulose nanofibers (CNF) also called microfibrillated cellulose (MFC), or bacterial nanocellulose, which refers to nano-structured cellulose produced by bacteria.
CNF is a material composed of nanosized cellulose fibrils with a high aspect ratio (length to width ratio). Typical fibril widths are 5–20 nanometers with a wide range of lengths, typically several micrometers. It is pseudo-plastic and exhibits thixotropy, the property of certain gels or fluids that are thick (viscous) under normal conditions, but become less viscous when shaken or agitated. When the shearing forces are removed the gel regains much of its original state. The fibrils are isolated from any cellulose containing source including wood-based fibers (pulp fibers) through high-pressure, high temperature and high velocity impact homogenization, grinding or microfluidization (see manufacture below).
Nanocellulose can also be obtained from native fibers by an acid hydrolysis, giving rise to highly crystalline and rigid nanoparticles which are shorter (100s to 1000 nanometers) than the nanofibrils obtained through homogenization, microfluiodization or grinding routes. The resulting material is known as cellulose nanocrystal (CNC).
History and terminology
The terminology microfibrillated/nanocellulose or (MFC) was first used by Turbak, Snyder and Sandberg in the late 1970s at the ITT Rayonier labs in Whippany, New Jersey, USA to describe a product prepared as a gel type material by passing wood pulp through a Gaulin type milk homogenizer at high temperatures and high pressures followed by ejection impact against a hard surface.
The terminology first appeared publicly in the early 1980s when a number of patents and publications were issued to ITT Rayonier on a new nanocellulose composition of matter. In later work Herrick[who?] at Rayonier also published work on making a dry powder form of the gel. Rayonier has been one of the world’s premier producers of purified pulps interested in creating new uses and new markets for pulps and not to compete with new customers. Thus, as the patents issued, Rayonier gave free license to whoever wanted to pursue this new use for cellulose. Rayonier, as a company, never pursued scale-up. Rather, Turbak et al. pursued 1) finding new uses for the MFC/nanocellulose. These included using MFC as a thickener and binder in foods, cosmetics, paper formation, textiles, nonwovens, etc. and 2) evaluate swelling and other techniques for lowering the energy requirements for MFC/Nanocellulose production. After ITT closed the Rayonier Whippany Labs in 1983–84, Herric worked on making a dry powder form of MFC at the Rayonier labs in Shelton, Washington, USA
In the mid 1990s the group of Taniguchi and co-workers and later Yano and co-workers pursued the effort in Japan. and a host of major companies, see numerous U.S. patents issued to P&G, J&J, 3M, McNeil, etc. using U.S. patent search under inventor name Turbak search base.
Manufacture
Nanocellulose, which is also called cellulose nanofibers (CNF), microfibrillated cellulose (MFC) or cellulose nanocrystal (CNC), can be prepared from any cellulose source material, but woodpulp is normally used.
The nanocellulose fibrils may be isolated from the wood-based fibers using mechanical methods which expose the pulp to high shear forces, ripping the larger wood-fibres apart into nanofibers. For this purpose, high-pressure homogenizers, ultrasonic homogenizers,[better source needed] grinders or microfluidizers can be used. The homogenizers are used to delaminate the cell walls of the fibers and liberate the nanosized fibrils. This process consumes very large amounts of energy and values over 30 MWh/tonne are not uncommon.
To address this problem, sometimes enzymatic/mechanical pre-treatments and introduction of charged groups for example through carboxymethylation or TEMPO-mediated oxidation are used. these pre-treatments can decrease energy consumption below 1 MWh/tonne.
Cellulose nanowhiskers are rodlike highly crystalline particles (relative crystallinity index above 75%) with a rectangular cross section. They are formed by the acid hydrolysis of native cellulose fibers commonly using sulfuric or hydrochloric acid. Amorphous sections of native cellulose are hydrolysed and after careful timing, crystalline sections can be retrieved from the acid solution by centrifugation and washing. Their dimensions depend on the native cellulose source material, and hydrolysis time and temperature.
In April 2013 breakthroughs[clarification needed] in nanocellulose production were announced at an American Chemical Society conference.
At ICAR-Central Institute for Research on Cotton Technology, Mumbai, India, a novel chemo-mechanical process for production of nanocellulose from cotton linters has been developed in the year 2013. To demonstrate this technology to the industrial users, a nanocellulose pilot plant is now operational at this Institute in Mumbai with a capacity of 10 kg per day. This facility was inaugurated in 2015.
Structure and properties
Dimensions and crystallinity
The ultrastructure of nanocellulose derived from various sources has been extensively studied. Techniques such as transmission electron microscopy (TEM), scanning electron microscopy (SEM), atomic force microscopy (AFM), wide angle X-ray scattering (WAXS), small incidence angle X-ray diffraction and solid state 13C cross-polarization magic angle spinning (CP/MAS), nuclear magnetic resonance (NMR) and spectroscopy have been used to characterize typically dried nanocellulose morphology.
A combination of microscopic techniques with image analysis can provide information on fibril widths, it is more difficult to determine fibril lengths, because of entanglements and difficulties in identifying both ends of individual nanofibrils.[page needed] Also, nanocellulose suspensions may not be homogeneous and can consist of various structural components, including cellulose nanofibrils and nanofibril bundles.
In a study of enzymatically pre-treated nanocellulose fibrils in a suspension the size and size-distribution were established using cryo-TEM. The fibrils were found to be rather mono-dispersed mostly with a diameter of ca. 5 nm although occasionally thicker fibril bundles were present. By combining ultrasonication with an “oxidation pretreatment”, cellulose microfibrils with a lateral dimension below 1 nm has been observed by AFM. The lower end of the thickness dimension is around 0.4 nm, which is related to the thickness of a cellulose monolayer sheet.
Aggregate widths can be determined by CP/MAS NMR developed by Innventia AB, Sweden, which also has been demonstrated to work for nanocellulose (enzymatic pre-treatment). An average width of 17 nm has been measured with the NMR-method, which corresponds well with SEM and TEM. Using TEM, values of 15 nm have been reported for nanocellulose from carboxymethylated pulp. However, thinner fibrils can also be detected. Wågberg et al. reported fibril widths of 5–15 nm for a nanocellulose with a charge density of about 0.5 meq./g. The group of Isogai reported fibril widths of 3–5 nm for TEMPO-oxidized cellulose having a charge density of 1.5 meq./g.
Pulp chemistry has a significant influence on nanocellulose microstructure. Carboxymethylation increases the numbers of charged groups on the fibril surfaces, making the fibrils easier to liberate and results in smaller and more uniform fibril widths (5–15 nm) compared to enzymatically pre-treated nanocellulose, where the fibril widths were 10–30 nm. The degree of crystallinity and crystal structure of nanocellulose. Nanocellulose exhibits cellulose crystal I organization and the degree of crystallinity is unchanged by the preparation of the nanocellulose. Typical values for the degree of crystallinity were around 63%.
Viscosity
The unique rheology of nanocellulose dispersions was recognized by the early investigators. The high viscosity at low nanocellulose concentrations makes nanocellulose very interesting as a non-caloric stabilizer and gellant in food applications, the major field explored by the early investigators.
The dynamic rheological properties were investigated in great detail and revealed that the storage and loss modulus were independent of the angular frequency at all nanocellulose concentrations between 0.125% to 5.9%. The storage modulus values are particularly high (104 Pa at 3% concentration) compared to results for cellulose nanowhiskers (102 Pa at 3% concentration). There is also a particular strong concentration dependence as the storage modulus increases 5 orders of magnitude if the concentration is increased from 0.125% to 5.9%.
Nanocellulose gels are also highly shear thinning (the viscosity is lost upon introduction of the shear forces). The shear-thinning behaviour is particularly useful in a range of different coating applications.
Mechanical properties
Crystalline cellulose has interesting mechanical properties for use in material applications. Its tensile strength is about 500MPa, similar to that of aluminium. Its stiffness is about 140–220 GPa, comparable with that of Kevlar and better than that of glass fiber, both of which are used commercially to reinforce plastics. Films made from nanocellulose have high strength (over 200 MPa), high stiffness (around 20 GPa) and high strain[clarification needed] (12%). Its strength/weight ratio is 8 times that of stainless steel. Fibers made from nanocellulose have high strength (up to 1.57 GPa) and stiffness (up to 86 GPa).
Barrier properties
In semi-crystalline polymers, the crystalline regions are considered to be gas impermeable. Due to relatively high crystallinity, in combination with the ability of the nanofibers to form a dense network held together by strong inter-fibrillar bonds (high cohesive energy density), it has been suggested that nanocellulose might act as a barrier material. Although the number of reported oxygen permeability values are limited, reports attribute high oxygen barrier properties to nanocellulose films. One study reported an oxygen permeability of 0.0006 (cm3 µm)/(m2 day kPa) for a ca. 5 µm thin nanocellulose film at 23 °C and 0% RH. In a related study, a more than 700-fold decrease in oxygen permeability of a polylactide (PLA) film when a nanocellulose layer was added to the PLA surface was reported.
The influence of nanocellulose film density and porosity on film oxygen permeability has recently been explored. Some authors have reported significant porosity in nanocellulose films, which seems to be in contradiction with high oxygen barrier properties, whereas Aulin et al. measured a nanocellulose film density close to density of crystalline cellulose (cellulose Iß crystal structure, 1.63 g/cm3) indicating a very dense film with a porosity close to zero.
Changing the surface functionality of the cellulose nanoparticle can also affect the permeability of nanocellulose films. Films constituted of negatively charged cellulose nanowhiskers could effectively reduce permeation of negatively charged ions, while leaving neutral ions virtually unaffected. Positively charged ions were found to accumulate in the membrane.
Multi-Parametric Surface Plasmon Resonance is one of the methods to study barrier properties of natural, modified or coated nanocellulose. The different antifouling, moisture, solvent, antimicrobial barrier formulation quality can be measured on the nanoscale. The adsorption kinetics as well as the degree of swelling can be measured in real-time and label-free.
Foams
Nanocellulose can also be used to make aerogels/foams, either homogeneously or in composite formulations. Nanocellulose-based foams are being studied for packaging applications in order to replace polystyrene-based foams. Svagan et al. showed that nanocellulose has the ability to reinforce starch foams by using a freeze-drying technique. The advantage of using nanocellulose instead of wood-based pulp fibers is that the nanofibrills can reinforce the thin cells in the starch foam. Moreover, it is possible to prepare pure nanocellulose aerogels applying various freeze-drying and super critical CO
2 drying techniques. Aerogels and foams can be used as porous templates. Tough ultra-high porosity foams prepared from cellulose I nanofibrill suspensions were studied by Sehaqui et al. a wide range of mechanical properties including compression was obtained by controlling density and nanofibrill interaction in the foams. Cellulose nanowhiskers could also be made to gel in water under low power sonication giving rise to aerogels with the highest reported surface area (>600m2/g) and lowest shrinkage during drying (6.5%) of cellulose aerogels. In another study by Aulin et al., the formation of structured porous aerogels of nanocellulose by freeze-drying was demonstrated. The density and surface texture of the aerogels was tuned by selecting the concentration of the nanocellulose dispersions before freeze-drying. Chemical vapour deposition of a fluorinated silane was used to uniformly coat the aerogel to tune their wetting properties towards non-polar liquids/oils. The authors demonstrated that it is possible to switch the wettability behaviour of the cellulose surfaces between super-wetting and super-repellent, using different scales of roughness and porosity created by the freeze-drying technique and change of concentration of the nanocellulose dispersion. Structured porous cellulose foams can however also be obtained by utilizing the freeze-drying technique on cellulose generated by Gluconobacter strains of bacteria, which bio-synthesize open porous networks of cellulose fibers with relatively large amounts of nanofibrills dispersed inside. Olsson et al. demonstrated that these networks can be further impregnated with metalhydroxide/oxide precursors, which can readily be transformed into grafted magnetic nanoparticles along the cellulose nanofibers. The magnetic cellulose foam may allow for a number of novel applications of nanocellulose and the first remotely actuated magnetic super sponges absorbing 1 gram of water within a 60 mg cellulose aerogel foam were reported. Notably, these highly porous foams (>98% air) can be compressed into strong magnetic nanopapers, which may find use as functional membranes in various applications.
Surface modification
The surface modification of nanocellulose is currently receiving a large amount of attention. Nanocellulose displays a high concentration of hydroxyl groups at the surface which can be reacted. However, hydrogen bonding strongly affects the reactivity of the surface hydroxyl groups. In addition, impurities at the surface of nanocellulose such as glucosidic and lignin fragments need to be removed before surface modification to obtain acceptable reproducibility between different batches.
Safety aspects
Health, safety and environmental aspects of nanocellulose have been recently evaluated. Processing of nanocellulose does not cause significant exposure to fine particles during friction grinding or spray drying. No evidence of inflammatory effects or cytotoxicity on mouse or human macrophages can be observed after exposure to nanocellulose. The results of toxicity studies suggest that nanocellulose is not cytotoxic and does not cause any effects on inflammatory system in macrophages. In addition, nanocellulose is not acutely toxic to Vibrio fischeri in environmentally relevant concentrations.
Applications
The properties of nanocellulose (e.g. mechanical properties, film-forming properties, viscosity etc.) makes it an interesting material for many applications and the potential for a multibillion-dollar industry.
Paper and paperboard
There is potential of nanocellulose applications in the area of paper and paperboard manufacture. Nanocelluloses are expected to enhance the fiber-fiber bond strength and, hence, have a strong reinforcement effect on paper materials. Nanocellulose may be useful as a barrier in grease-proof type of papers and as a wet-end additive to enhance retention, dry and wet strength in commodity type of paper and board products. It has been shown that applying CNF as a coating material on the surface of paper and paperboard improves the barrier properties, especially air resistance. It also enhances the structure properties of paperboards (smoother surface).
Nanocellulose can be used to prepare flexible and optically transparent paper. Such paper is an attractive substrate for electronic devices because it is recyclable, compatible with biological objects, and easily degrades when disposed of.
Like resin-free lignocellulose fiberboard which are produced using wet process, high tough cellulose nanofiber board with thickness of 3 mm was also introduced by Yousefi et al., 2018.
Composite
As described above the properties of the nanocellulose makes an interesting material for reinforcing plastics. Nanocellulose has been reported to improve the mechanical properties of, for example, thermosetting resins, starch-based matrixes, soy protein, rubber latex, poly(lactide). The composite applications may be for use as coatings and films, paints, foams, packaging.
Food
Nanocellulose can be used as a low calorie replacement for today’s carbohydrate additives used as thickeners, flavour carriers and suspension stabilizers in a wide variety of food products and is useful for producing fillings, crushes, chips, wafers, soups, gravies, puddings etc. The food applications were early recognised as a highly interesting application field for nanocellulose due to the rheological behaviour of the nanocellulose gel.
Hygiene and absorbent products
Applications in this field include: Super water absorbent material (e.g. for incontinence pads material), nanocellulose used together with super absorbent polymers, nanocellulose in tissue, non-woven products or absorbent structures and as antimicrobial films.
Emulsion and dispersion
Nanocellulose has numerous applications as a food additive, and in the general area of emulsion and dispersion applications in other fields. Oil in water applications were early recognized. Early investigators had explored the area of non-settling suspensions for pumping sand, coal as well as paints and drilling muds.
Oil recovery
Hydrocarbon fracturing of oil-bearing formations is a potentially interesting and large-scale application. Nanocellulose has been suggested for use in oil recovery applications as a fracturing fluid. Drilling muds based on nanocellulose have also been suggested.
Medical, cosmetic and pharmaceutical
The use of nanocellulose in cosmetics and pharmaceuticals was also early recognized. A wide range of high-end applications have been suggested:
Freeze-dried nanocellulose aerogels used in sanitary napkins, tampons, diapers or as wound dressing
The use of nanocellulose as a composite coating agent in cosmetics e.g. for hair, eyelashes, eyebrows or nails
A dry solid nanocellulose composition in the form of tablets for treating intestinal disorders
Nanocellulose films for screening of biological compounds and nucleic acids encoding a biological compound
Filter medium partly based on nanocellulose for leukocyte free blood transfusion
A buccodental formulation, comprising nanocellulose and a polyhydroxylated organic compound
Powdered nanocellulose has also been suggested as an excipient in pharmaceutical compositions
Nanocellulose in compositions of a photoreactive noxious substance purging agent
Elastic cryo-structured gels for potential biomedical and biotechnological application.
Matrix for 3D cell culture
Other applications
As a highly scattering material for ultra-white coatings.
Activate the dissolution of cellulose in different solvents
Regenerated cellulose products, such as fibers films, cellulose derivatives
Tobacco filter additive
Organometallic modified nanocellulose in battery separators
Reinforcement of conductive materials
Loud-speaker membranes
High-flux membranes
Computer components
Capacitors
Lightweight body armour and ballistic glass
Corrosion inhibitors
Commercial Production
Although wood-driven nanocellulose was first produced in 1983 by Herrick and Turbak, its commercial production postponed till 2010, mainly due to the high production energy consumption and high production cost. Inventia Co. in Sweden was the first nanocellulose company established in 2010. Other first generation active companies are CelluForce (Canada), Nippon (Japan), Nano Novin Polymer Co. (Iran), Maine University (USA), VTT (Finland), Melodea (Israel), etc.
Source from Wikipedia